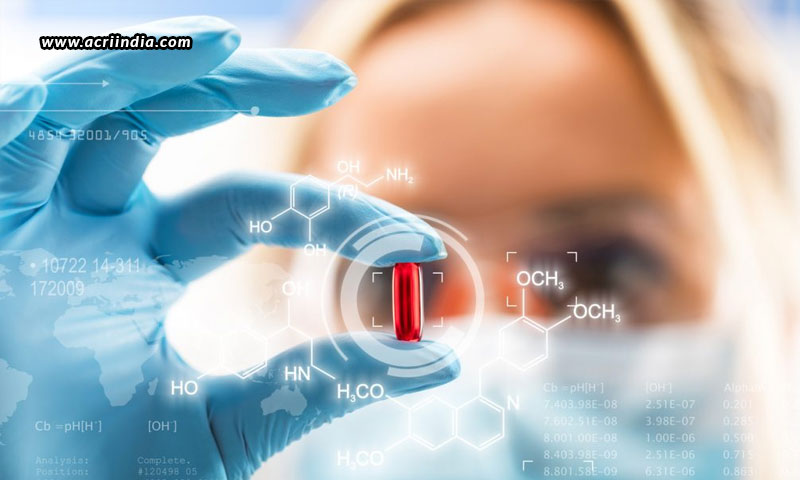
The drug development process is a long, expensive, and failure-prone process that requires cutting-edge skills. It takes around 8-12 years on an average for a new drug to be developed, going through a course of rigorous trials, which if cleared can be approved for human use. Only as many as 1-10 out of thousands of drugs are approved by the administration to be fit for human use! The rest of the drugs are all abandoned. Thus, drug development is a very crucial process that requires all the safety concerns considered and measured well before expecting it to become a success.
This crucial process of drug development also requires collaboration across multiple disciplines within the pharmaceutical industry, and among research laboratories, healthcare professionals, government regulators, and educational institutions. Without collaboration, nothing can be a success in the pharmaceutical industry.
Now, let us understand the process of drug development and approval. Drug development goes through three basic stages – drug discovery, full development, and clinical trials, before being considered for approval.
Drug discovery
Just like with anything else, there is a planned idea behind the development of drugs. A new idea is thought about, which is directed at chemically modifying a disease process. Considering the disease that needs to be treated, a new drug is developed, which is expected to react with a new molecular target within the human body. This idea is generated from thorough knowledge and complete understanding of the disease process, along with a continual involvement with research in the specific therapeutic area of interest.
Full development
The drug developed then goes through early trials in people who have developed the disease that is to be targeted. The study aims to find out if a drug behaves as it is expected to. Only a small dose of drug is tested onto a random number of people to check for any side effects. The test is done to find out if the drug is safe, has any side effects, and how the drug is eliminated from the body. Only those drugs that are found safe in measurable amounts within a certain amount of people are considered to be fully developed. However, they still need to go through a number of phases of clinical trials to be considered approved.
Clinical trials
After the drug has been considered safe to a certain extent, it is then moved on to the later stages of clinical trials for deeper study. The trials may be compared to other treatments that may be currently available. This comparison may find out which treatment works better for a particular type of disease. It will also let the researchers understand more about the side effects in detail, and how the treatment affects patients’ quality of life. Different doses may also be experimented. Different types of the same disease may be tried to be treated with the new drug. Once the drug is proved to be completely safe, the drug is licensed and approved to be marketable throughout the country.
This entire procedure takes somewhere around 8-12 years, right from the development of the drug to reach the shelf of the patients. A clinical research thus has to be properly designed and planned to provide reliable efficacy and safety data. The drug needs to be completely safe and effective to be approved by regulatory authorities and an Ethics Committee, which will permit the trial to be conducted at a particular institute. And, one institute that can provide you with the best clinical research training in Bangalore to help you achieve a successful career in clinical research – the hottest career today – is Avigna Clinical Research Institute, where you will be mentored by experienced professionals through personalized training and complete coaching.